
|
KERALA AGRICULTURAL UNIVERSITY
|
Choice of a fertilizer depends on unit cost of nutrient present in it and its agronomic efficiency under a given situation. Fertilizer is a valuable input and measures should be taken to reduce its losses and to increase its uptake and utilisation by the crop. Selecting a situation-specific fertilizer and choosing the time and method of application according to crop demand would minimize losses and increase its efficiency.
Nitrogenous fertilizers
Most crop plants recover only 25-35 per cent of the nitrogen applied as fertilizers. Losses occur by ammonia volatilisation, denitrification, immobilization to organic forms, leaching and run off. Utmost care should be bestowed in selecting the type of fertilizer as well as the timing and method of application.
Choice of the nitrogen fertilizer
1. In submerged rice soil, ammoniacal and ammonia producing fertilizers like urea are most suitable since ammonia is the most stable form of nitrogen under such conditions.
2. For acidic upland soils, ammoniacal fertilizers are most suitable during rainy season since ammonium is adsorbed on soil particles and hence leaching losses are reduced. Adsorbed ammonium is gradually released for nitrification and thus becomes available to crops for a longer period.
3. In highly acidic upland soils, urea is
preferred to ammonium sulphate as
the former is less acid forming.
4. In alkaline upland soils of low rainfall
regions, nitrate fertilizers
are preferred to
ammoniacal fertilizers or urea since ammonia may be lost by volatilization
under alkaline conditions.
Management of nitrogenous fertilizers
1. Almost all the nitrogenous fertilizers are highly amenable to losses and since
most of the crops require nitrogen during the
entire growth period, split application is necessary to ensure maximum
utilization by crops.
2. More number of splits may be given for long duration crops as well as
perennial crops.
3. Nitrogen losses from fertilizers are more in coarse textured soils with low
cation exchange capacity (CEC) than
in fine textured soils. Hence more number of splits is necessary to reduce loss
of fertilizer nitrogen from sandy and other light soils.
4. For medium duration rice varieties, nitrogenous fertilizers should be given in three splits, as basal, at maximum tillering and at panicle initiation stage.
5. In coarse textured sandy or loamy soils, the entire dose of nitrogenous fertilizers may be applied in 3-4 splits at different stages of growth of rice crop.
6. In areas where split application of nitrogen is not feasible due to water stagnation after planting/sowing, full dose of nitrogen as basal may be given in the form of neem coated or coal tar coated urea.
7. In double cropped wetlands, 50 per cent of N requirement of the first crop may be applied in the organic form.
8. As far as possible, liming should be done one or two weeks prior to the application of ammoniacal or ammonia forming fertilizer like urea since ammonia is likely to be lost by volatilization if applied along with lime.
9. Almost 70 per cent of N in urea applied
by broadcast to flooded soil is lost by
volatilization, immobilization and by
denitrification
Measures to reduce the loss of N from applied urea
1. Urea super granules or urea briquettes may be used in places where soil is
clayey and has cation exchange capacity more than 10 cmol (+) per kg of soil.
2. Sulphur or lac coated urea is suitable where soil is liable to intermittent
flooding and in situations where water management is difficult. This is more suitable
for direct sown crop.
3. Urea may be mixed with moist soil and kept for 24-48 hours before application
to the field. Alternatively, urea may be mixed with moist soil, made into balls of
about three inch diameter and dried under
shade. The balls may be placed deep into
subsoil.
4. Mixing urea with one fifth its weight of neem cake (5:1) prolongs the period of nitrogen availability to the crop.
5. For submerged soils, coating urea with coal tar and kerosene (100 kg urea is mixed with 2 kg coal tar dissolved in one litre kerosene) before mixing with neem cake is preferred to simple mixing with neem cake.
6. Coating urea with neem extract (containing about 5
per cent neem triterpenes) at 1 per cent rate and shade drying for 1
to 1.5 hours before applying in direct seeded puddled lowland rice increases
nitrogen use efficiency.
7. As far as possible, urea may be applied by deep placement or plough sole placement. Deep placement of prilled urea or super granules during the last ploughing followed by flooding and planting is beneficial in light soils. Urea briquettes or super granules may be placed between four hills of transplanted rice, whereas sulphur coated or lac coated urea may be broadcast on the surface.
8. Foliar spray of 5 per cent urea solution can be practised in situations where quick response to applied nitrogen is required. If power sprayers are used, the concentration may be increased to 15 per cent. Fresh urea should be used to avoid toxicity due to biuret.
Phosphatic fertilizers
Fertilizer phosphorus is an expensive input and its management poses serious problems due to several complexities in its behaviour in different types of soil. This often results in its poor recovery from applied fertilizers.
Choice of phosphatic fertilizer
1. In slightly acid, neutral or mildly alkaline soils, water soluble phosphatic
fertilizers are more suitable.
2. In wetland rice soils, water soluble phosphatic fertilizers are preferable as pH of most of the submerged soils is near neutral.
3. In strongly acidic soils whose pH does not rise above 5.5 to 6.0 even on
submergence, phosphatic fertilizers containing
citrate soluble form of P like basic slag, dicalcium
phosphate, steamed bone meal etc. are suitable.
4. For highly acidic upland soils or
submerged soils whose pH will not
rise above 5.5 even on submergence,
powdered rock phosphate is suitable. Soil acidity converts tricalcium phosphate
in rock phosphate to plant available monocalcium form.
5. For short duration crops where quick response is required, water soluble phosphatic fertilizers are most suitable.
6. For perennial crops like rubber, oil palm, coffee, tea, cardamom etc. phosphorus
in the form of rock phosphate can be
applied.
7. In black soil (Chittur taluk of Palakkad District) phosphatic fertilizers
containing water soluble phosphate like single superphosphate are most suitable.
Management of phosphatic fertilizers
1. Acid soils have to be amended with lime, dolomite or magnesium silicate and
alkali soils with iron pyrite or sulphur before application of phosphatic
fertili-zers. This will help to reduce fixation
and increase availability of P.
2. Surface application or broadcasting is
preferred for shallow rooted
crops whereas placement in the root zone is
advantageous in deep rooted crops.
3. Rock phosphates can be used advantageously in rice, grown in acid soils
during the virippu season. Powdered
rock phosphate may be applied and mixed
thoroughly with soil by ploughing. After two or three weeks, the field may
be flooded, worked up and planted with rice. Under this situation, phosphorus in rock phosphate gets converted to iron
phosphate, which on subsequent waterlogging becomes available to the rice crop.
4. Rock phosphate can be used successfully as a phosphatic source for leguminous crop since its root system can extract phosphorous from rock phosphate.
5. In single crop wetlands where rice is grown in the virippu season,
application of phosphatic fertilizers can be dispensed with for the rice crop, if the second
crop (usually legume or green manure) is given phosphatic fertilizers.
6. In case of rice-legume cropping sequence in acid soils, application of rock
phosphate to the pulse crop helps to skip phosphatic fertilizers in the
succeeding rice crop.
7. Since phosphorus requirement of seasonal crops is confined to the early stages, phosphatic fertilizers are to be applied at the time of seeding or planting. Top dressing of phosphatic fertilizer leads to wastage of the fertilizer nutrient. Further, excessive phosphates may lead to deficiency of micronutrients such as zinc, boron etc.
8. Under adverse soil conditions and where quick result is required, spraying water-soluble phosphatic fertilizers like triple superphosphate or hot water extract of superphosphate can be resorted to.
Potassium fertilizers
For most crops, potassium can be
supplied as muriate of potash. But in
crops like tobacco and potato, muriate of potash may cause chloride injury, reducing
quality of the produce. In such cases, K may be
applied as potassium sulphate.
Management of potassium fertilizers
1. In coarse textured soils and in heavy rainfall regions, potassium fertilizers should be applied in as many splits as possible, to reduce loss of potassium.
2. In fine textured soils, the entire dose of
potassium fertilizers may be applied
as basal.
3. In acid soils, potassium fertilizers should be applied only after lime application to prevent loss of potassium by leaching.
Lime
Acid soils are characterised by high saturation of the exchange complex with hydrogen and aluminium. Crops grown in such soils suffer due to unavailability of most plant nutrients, especially calcium. Application of liming materials increases the availability of nutrients and alleviates Ca deficiency.
Liming materials
Burnt lime [CaO], slaked lime [Ca
(OH)2], powdered limestone
[CaCO3] and dolomite [CaMg
(CO3)2] are some of the
materials used as sources of calcium.
Management
1. In acidic submerged soils, flooding brings about rise in soil pH and hence response to lime is less marked.
2. Legumes are benefitted most by liming.
3. For better results, liming materials should be incorporated into the soil.
4. For seasonal crops and in situations where immediate results are required, burnt
lime or slaked lime may be used. For
perennial crops, powdered lime stone or dolomite is sufficient.
5. Extreme care should be taken while broadcasting burnt lime and slaked lime as they can cause scorching of leaves.
6. In case of wetland rice, drain the field prior to lime application and reflood after 24 hours. Flushing the soil by sequential flooding and draining will help to wash out the displaced acid from the soil. 7. In extreme case of calcium deficiency, 1 per cent solution of calcium chloride may be applied by foliar spraying.
True honey bees belong to the family Apidae and genus Apis. They are social insects living in colonies. A colony consists of a queen, several thousand workers and a few hundred drones. There is division of labour and specialization in the performance of various functions. They build nests (combs) with wax secreted from the wax glands of worker bees. The bees use these cells to rear their brood and store food. Honey is stored in the upper part of the comb; beneath it are rows of pollen storage cells, worker brood cells and drone brood cells in that order. Some Apis species build single comb in open, while others build multiple combs in dark cavities.
Species of honeybees
There are four species of honeybees in India. They are:
Rock bee (Apis dorsata): They are giant bees found all over India in sub-mountainous regions up to an altitude of 2700 m. They build single comb nests with an area up to 1 m2 or more. They are good honey gatherers with an average yield of 50-80 kg per colony.
Little bee (Apis florea): They are the smallest of the true honeybees found in plains of India up to the altitude of 500 m. They build single vertical combs. They are poor honey yielders and yield about 200-900 g of honey per colony.
Indian bee (Apis cerana indica): They are the domesticated species, which construct multiple parallel combs with an average honey yield of 6-8 kg per colony per year.
European bee/Italian bee (Apis mellifera): They are also similar in habits to Indian bees and build parallel combs. They are bigger than all other honeybees except Apis dorsata. The average production per colony is 25-40 kg.
Stingless bee (Trigona iridipennis):
In
addition to the above, another species is also present in Kerala known as stingless
bees. They are not truly stingless, but the sting
is poorly developed. They make nests in the ground, hollows of trees, bamboo, rocks
or cracks of walls. Honey and brood cells are separate in the nest. They are efficient
pollinators. They yield 300-400 g of
honey per year.
Swarming
Swarming is the natural instinct of honeybees to reproduce its colonies. By swarming, strong colonies are divided naturally. It occurs mostly when the colony population is at its peak. Some of the several reasons for swarming are sudden honey flow, sudden failure of queen to lay eggs, congestion in the colony, want of breeding space, bad ventilation etc. Dividing the colonies or keeping young queen or preventing over crowding of bees or adding new combs can prevent swarming.
Absconding
Absconding is the total desertion of colony from its nest due to incidence of disease / pest attack, too much interference by human beings or robbing of honey by bees from other colonies. Proper hive management can prevent it.
Communication
The worker bees communicate with other bees about the exact location of nectar,
pollen, water, next nesting site etc. by
means of dances. Round dance is performed when the food is located within 100 m from hive
and wagtail dance to communicate the
location of food source when it is more
than 100 m away from the hive.
Bee space
It is the space large enough to permit the free passage for worker bees but too small to encourage bees building a comb and too large for bees to deposit propolis in it.
Indian bee (Apis cerana indica)
This is the domesticated hive bee in Kerala. A colony consists of a queen, 20,000 to 30,000 workers and a few drones. This species has gentle temperament and responds to smoking. Lack of flora leads to absconding by bees. It also has a strong tendency for swarming. It yields 8-10 kg of honey per colony per year.
Bee-box
ISI Type-A box is recommended for the State of Kerala. A division board may
be added to the bee box for adjusting the
internal space depending on the strength of the colony. It can be procured from
beekeepers. Wild feral colonies can be hived.
Beekeepers in different regions use
local hives made of low cost wood. The wood should not have a strong smell. Kail
(Pinus excelsa), teak (Tectona
grandis), toon (Toona ciliata) anjili
(Artocarpus hirsutus), punna (Calophyllum inophyllum)
etc., are some of the suitable woods. The hives
should be preferably painted white on outside to
protect the timber from weathering.
Hiving wild colony
It is done during evening hours. Smoke the colony slightly, cut out the combs one by one and tie to the brood frames with plantain fibre. Arrange them in the box.
Location of beehives
The apiary must be located in well-drained open area, preferably near orchards, with profuse source of nectar, pollen and water. Windbreaks may be provided by planting shrubs, flowering plants and also creepers like antigonon. Shade must also be provided. Ant wells are fixed around the hive stand. The colonies must be directed towards east, with slight changes in the directions of the bee box as a protection from rain and sun. Keep the colonies away from the reach of cattle, other animal, busy roads and streetlights.
Management of colonies
Inspect the beehives at least once in a week during brood rearing / honey-flow
seasons preferably during the morning hours. Bright, warm and calm days are suitable.
If sunlight falls directly on the beehive spread cloth or a towel over the same. Look
for freshly laid eggs to ensure that the colonies are healthy. Clean the hive in the
following sequence, the roof, super/supers, brood
chambers and floorboard. Observe the colonies regularly for the presence of healthy
queen, brood development, storage of honey and pollen, presence of queen cells, bee
strength and growth of drones. Look for the
infestation by any of the following bee enemies.
Wax moth (Galleria mellonella): Remove all the larvae and silken webbings from the combs, corners and crevices of bee box.
Wax beetles (Platybolium sp.):
Collect and destroy the adult beetles.
Mites: Clean the frame and floorboard with cotton swabs moistened with freshly
made potassium permanganate solution. Repeat until no mites are seen on the floorboard.
Diseases: The dead larvae due to Thai
sac brood virus (TSBV) in the comb cells may be removed and destroyed.
Management during lean season
Remove the supers and arrange the available healthy broods compactly in the brood chamber. Provide division board, if necessary. Destroy queen cells and drone cells, if noted. Provide sugar syrup (1:1) @ 200 g sugar per colony per week for Indian bees. Feed all the colonies in the apiary at the same time to avoid robbing.
Management during honey flow season
Keep the colony in sufficient strength before honey-flow season. Congestion in the hive must be avoided and surplus honeybees are drawn to supers. Provide maximum space between the first super and the brood chamber and not above the first super. Place queen excluder sheets in between brood and super chamber to confine the queen to brood chamber. Examine the colony once in a week and frames full of honey should be removed to the sides of the super and such frames can be raised from brood to super chamber. The frames, which are three-fourth filled with honey or pollen and one-fourth with sealed brood should be taken out of brood chamber and in its place empty combs or frames with foundation is added. The frame with comb foundation should be placed next to the brood nest. The combs, which are completely sealed, or two-third capped may be taken out for extraction of honey and returned to supers after honey extraction. This helps the colonies to activate the bees to collect and store more honey. Two or three such extractions are possible during a surplus flow. Extraction of uncapped honey will result in fermentation. Honey extraction, after the flow is over, should be avoided to save the bee colonies from robbing. Care should be taken to retain sufficient combs with honey in the brood chamber or reduce the lean period.
Migratory bee keeping
The moving of bee colonies from one place to another to capture increased nectar flow of a particular flora is called migratory beekeeping. Copious flow of extra floral nectar available on rubber trees during January-April is exploited by shifting bee colonies to these plantations during this period.
Similar practice is done in cashew plantations and in other orchards too. Maintaining bee colonies in orchards will increase the yield, since pollination is more efficient in such orchards.
Shifting of colonies is done after sun set. Colonies should be prepared as follows. Extract available honey and fasten all the weak combs to frames with plantain fibres. Secure the frames to the chamber with packing. Close the bee entrance with cotton. Then secure the bee-box (floorboard, brood chamber, supers and roof) firmly with strong threads. Do not tilt or topple beehives while stacking them in the conveyance or during transit. Avoid strong jerks and shocks while transporting.
Set up the beehives as described above at the new site. Inspect the condition of combs and tighten loose threads, if any. This inspection should be done only in dim light. Next morning remove the cotton plug at bee entrance. Later provide comb foundation sheets, if necessary and provide sufficient space for storage of honey.
Extraction of honey
Honey is extracted only from super combs using honey extractor. The sealing of cells on combs is removed with sharp knife before placing in the extractor. Extractor should be worked slowly at the beginning and at about 150 rpm at the end for about 1 to 2 minutes. Then the sides of the frames are reversed and the extractor is again worked. Extracted honey is filtered through muslin cloth. Providing a bee escape between the brood and super on the day prior to honey extraction keeps the bees away from the super. Remove the escape soon after honey extraction.
Processing of honey
Heat the honey to 45ºC by keeping it in a water bath. Sieve it to remove wax
particles, debris, dust and pollen. Again heat it to a
temperature of 65ºC in water bath and
maintain it for 10 minutes. Then cool and filter it in
80-mesh muslin and store in glass,
porcelain, earthenware, enamelware or stainless
steel containers. Bulk storing can be made in mild steel containers lined with bee wax.
Italian bee (Apis mellifera)
It is a native of Europe introduced to Himachal Pradesh and Punjab during 1962-64 and introduced to Kerala on a trial basis from Haryana in November 1992. It maintains a prolific queen, swarms less, has gentle temperament and is a good honey-gatherer. It is known to be resistant to TSBV. A healthy colony may contain 60,000 to 80,000 worker bees. The following modifications are to be followed in beekeeping with Italian bees.
Bee-box
Langstroth beehive with ten frames each in brood and super chambers and a division brood chamber is recommended. The brood and super chambers are of the same size.
Procuring bee colonies
Colonies can be obtained either by dividing existing colonies or by buying from other agencies.
Location of beehives
Follow the practices as in Indian bees, but use a strong four-legged stand well protected from ants and other crawling insects by providing ant wells.
Management of colonies
Apart from the management practices followed for Indian bee, the practices
as mentioned below may be followed.
Sources of pure water should be available near the apiary. Stagnant water or water in a container is not appropriate because it can spread nosema disease. Flowing water near the apiary should serve as a good source. As an alternative, water trickling from a container set on a stand and falling on a slanting wooden plank can be provided.
During the brood rearing season (growth period) from October to January, replacement of old queens by young healthy ones, uniting the weak colonies and giving supplementary feeding as and when required should be done. Colonies should be provided with enough space for brood rearing and food storage, by giving comb foundation sheets one at a time.
In areas where queen mating is a
problem, especially when only a few
colonies are kept in isolated pockets,
the colony with virgin queen is to be transferred to areas where more number of colonies
are kept so as to ensure the availability of queen in sufficient numbers and afterwards
returned to the former apiary.
During honey flow season (January-April), provide raised combs in the super and the number of combs to be added depends on the strength of the colony. Only ripe honey is harvested when two-third of the comb cells are capped so that honey contains less than 20 per cent moisture. Care should be taken to see that the bee colonies are not stripped of all the honey stores. Enough stores of honey should be ensured in the hive at the end of honey flow for use during the following lean period. For migratory bee keeping, follow the practices as adopted for Indian bees.
Extraction of honey
The sealing of comb is removed with a sharp knife and the extraction done in
an extractor designed for langstroth size frames. Extracted honey is filtered through a
coarse cloth to remove the impurities.
Processing of honey
To be done as described under Indian bees. During the lean season (May-September), remove the super chambers, arrange the available healthy brood combs in the brood chamber and use division boards to restrict the space. Provide artificial feeding once in a week by way of 1:1 sugar syrup in water. Each colony may require syrup prepared from 500-750 g sugar a week depending on the size of the colony and availability of stored food. When there is dearth of natural source, pollen substitutes may be provided in the colony.
Pests and diseases
Brood mite (Tropilaelaps clareae): Infests
the brood and the infestation is severe during the major brood rearing season
(October-January). These ectoparasites feed on the haemolymph of developing broods slowly
killing them. Dusting sulphur on the topbars of the frames @ 200 mg/ frame at
7-14 days interval during brood rearing season is
very effective in checking the infestation.
Yellow-banded wasp (Vespa cincta): These predatory wasps catch the bees from both the hive entrance and inside the hives. Locating and destroying their nests by burning or insecticidal usage is an effective control measure.
Wax moth (Galleria mellonella):
Infests weak and unattended colonies. Proper cleaning of the hives periodically and keeping
the hives without cracks and crevices can avoid infestation.
Black ants: Various species of black ants intrude beehives and take away honey and pollen and kill the brood and bees, which may lead to absconding of colony. The apiary should be kept clean and the ant nests destroyed by insecticidal applications. Ant wells should be provided for the beehive stands.
Red tree-ant (Oecophyla smaragdina)
If not protected properly, the red tree-ants can cause considerable damage to the bees and the brood. The bees that come in contact with the ground are attacked and killed by the ants and dragged to their nests by a number of ants. In the apiary, if the branch of a tree with these ants happens to come in contact with the hive, the entire colony is attacked and destroyed. Providing ant wells will keep away the ants. Care should be taken not to keep the colonies near or under the trees having ant nests.
Bee-eater bird (Merops orientalis)
Thai sac brood virus
Symptoms
All the larval instars are susceptible to
the disease, earlier instars being more
susceptible. Affected larvae appear
slightly plumby compared to healthy ones when examined on taking out of the comb
cells. The infected larvae seen stretched on their back in the cells with the head directed
outwards and turned upwards like the
prow of a boat. The dead larvae look like a sac filled with milky white fluid when lifted
up and it ruptures even with the slight pressure releasing the milky fluid. The
cadavers change their colour from white to pale yellow and sunk down to the floor of the
cell and dry up in 10-15 days as brownish black
boat shaped scales, which are easily
removable from the cells. 2. Dead larvae are seen lying stretched
out on their back on the floor of brood cells and look like a sac filled with milky
white fluid when lifted up.
3. Appearance of dead larvae strewn on
the floorboards, hive entrance or on the floor near the hive.
4. Mottled appearance of brood combs
with uncapped cells interspersed with capped cells or cells with perforated capping.
5. Appearance of more and more dead
larvae left within the cells without
being ejected by the worker bees. Being a virus disease there is no
known remedy. However, the following measures may help in minimizing the possibilities of
further spread: a) Keep colonies strong; c) avoid procurement of colonies or
swarms from infected areas.
These predatory birds do much harm in certain localities. They pick the bees on
wings and 30-43 honeybees have been found in the stomach of a bird. Attack by these birds
is mostly seen during December-January. These birds are also very useful in
keeping down the insect population in a locality
and hence no large-scale measures against them can be recommended. Scaring them
away from apiaries is suggested.
The sequence of visible symptoms found in the field is:
1. Presence of unsealed cells in brood area containing diseased larvae with their
head directed outwards like the prow of a boat.
6. Appearance of sac like remnants of dead larvae within the cells.
7. Lack of cleaning activity within the hive.
8. Decrease in egg laying rate and irregular placement of eggs.
9. Decrease in foraging activity and
presence of idling workers inside the hive.
10. Dwindling of bee population of the colony.
11. Desertion of infected hives by the bees causing total loss to the apiary.
Management
b) avoid exchange of hive parts, combs etc. from infected colonies to healthy colonies;
SERICULTURE
(Ad hoc recommendation)
Moriculture
Mulberry can be grown under various climatic conditions ranging from temperate to tropical. Its growth depends on many climatic conditions such as temperature, humidity, rainfall etc. A temperature range of 24-28ºC, humidity range of 65-80 per cent and 600-2500 mm rainfall are ideal for optimum growth of mulberry. The soil should be deep, fertile, well drained, clay loam to loam and with good moisture holding capacity. Slightly acidic (6.2-6.8 pH) soil free from injurious salts is ideal for the growth of mulberry.
Land preparation
The field is levelled and ploughed deeply before the onset of monsoon. FYM may be applied @ 10 t ha-1 for the rainfed crop and 20 t ha-1 for the irrigated crop during land preparation.
Method of planting and spacing
(1) Pit system (rainfed crop): Spacing 75 cm x 75 cm (pit size 30 cm x 30 cm x
30 cm)(2) Row system (irrigated crop):
Spacing 60 cm x 60 cm (ridges and furrows)
Planting material
The variety K2 gives higher yield and better quality leaves. Cuttings must be prepared from shoots of proper maturity (6-8 months) and thickness with well developed buds. Cuttings of 7-10 cm length and pencil thickness with 3 or 4 active buds are ideal.
Planting
For irrigated crop, two cuttings should be planted at each spot along the margin of the ridge.
For rainfed crop, three cuttings are to be planted per pit in a triangular manner with a distance of 15 cm, keeping only one bud exposed.
Maintenance of the garden
(1st year)
After 8 months of planting, 50 kg each of N,
P2O5 and K2O should be applied per
ha after weeding. First harvest can be taken six months after planting by leaf picking.
Second dose of 50 kg N per ha should
be applied 8 weeks after the first leaf harvest. Two more crops can be taken at an
interval of 3 months, by leaf picking.
Manuring
For rainfed crop apply FYM @ 10 t ha-1 as a basal dose and topdress every year
at the time of annual pruning. Fertilizers are applied @ 130:65:65 kg
ha-1 of
N:P2O5:K2O in two split doses. For irrigated crop, FYM
is given @ 20 t ha-1 as basal dose.
Fertilizers are applied @ 300:120:120 kg
ha-1 of N: P2O5:
K2O in five split doses.
Pruning
For rainfed crop, bottom pruning is done in May-June. Two top clippings in August/ September and December/January are also practised. Middle pruning is done in October/November. For irrigated crop, bottom pruning at 15-30 cm height in May, two top clippings in August and December and two middle pruning at 60 cm height in October and February/March are practised.
Pests
Tussock caterpillars (Euproctis fraterna)
Larvae eat the leaves of the mulberry plant. Their incidence is frequent during
March to August. Collection and
destruction of egg masses and spraying 1 per cent
DDVP are effective. Waiting period is 3 days.
Jassids (Empoasca flarescens)
Greenish hoppers feed on the underside of the leaf, suck sap and cause hopper burn. Spraying 0.05 per cent dimethoate is effective. Waiting period is 10 days.
Thrips
These are frequent during summer season. Attack is severe in rainfed gardens. Spraying 0.02 per cent DDVP is effective. Waiting period is 3 days.
Mealy bugs (Maconelliococcus hirsutus)
It causes `tukra disease'. The affected leaves show curling and stunted growth
at the growing point.
Scale insect
When attack is severe, branches dry and become yellow. Spraying lime sulphur solution is effective.
Leaf eating caterpillar (Diacrisia obliqua)
Appears frequently between November and January. Collection and destruction of egg masses, deep ploughing and flood irrigation to kill the pupae and application of 0.05 per cent DDVP on the leaves can prevent the attack.
Root knot disease (Meloidogyne incognita)
Common in sandy loam type of soil
under irrigated conditions. Controlled by
applying neem oil cake at the rate of 400
kg per ha per year in four equal split doses.
Diseases
Powdery mildew (Phyllactina corylea)
It is more common during November- February. White powdery patches appear on the lower side of the leaves.
Leaf rust (Ceratelicum fici)
The attacked portion of the leaves have whitish brown pustules on both sides, are deformed and non-nutritive. Infection is more in November-February. This can be controlled by spraying carbendazim 0.05 per cent or tridemorph 0.08per cent.
Leaf spot (Cercospora moricola)
Diseased leaves have a number of circular or irregular brownish black spots of varying size. Infection is more common in rainy season. This can be controlled by spraying 0.05per cent of carbendazim.
Yield
Rainfed crop : 12000-15000 kg/ha/year
Irrigated crop : 25000-30000 kg/ha/year
Silkworm rearing
Requirements for silkworm rearing
1. Good quality mulberry leaves
2. Rearing house of approximately 20
m2 for 100 dfls (disease free layings), with
good ventilation, mild temperature (24-28ºC) and humidity (65-85
per cent).
3. Rearing equipments like chawki stand (one), wooden trays (10), rearing racks (5), chopping board (one) and knife, wooden / bamboo rearing trays (50), chandrika / netrika (mountage) (40), leaf chamber, feeding stands, ant wells, rocker sprayer, wet and dry bulb thermometer and materials like formaldehyde / bleaching powder, paraffin paper, cleaning nets, foam rubber strips, and RKO powder are required.
Rearing techniques
Disinfect the rearing house and equipments two-three days before rearing to prevent silkworm disease. First, wash the rearing house and the equipments with 2 per cent bleaching powder. Then spray the room and equipments with 5 per cent bleaching powder or 2 per cent formaldehyde. Keep the rearing houses closed for 24 hours for the fumes to get diffused.
First incubate the dfls (egg card) at a
temperature of 24-26ºC and RH of 75-80 per
cent, one day prior to hatching (blue egg stage); cover the eggs with black
paper (black boxing). Next day morning, open it
and expose to diffused sunlight. As the
larvae emerge out, fresh tender leaves
collected from the plant are chopped into
0.5 mm x 0.5 mm size and sprinkled over
the hatched larvae. After half an hour, transfer the larvae to the paraffin paper spread in
the chawky trays (wooden trays) using fine brush. Provide wet foam strips around
and prepare a compact bed. Give another feeding in the bed. Cover with paraffin paper
and stack the trays one over the other on the stand. Upto 20 layings can be brushed in
a tray of 90 cm x 60 cm.

At the end of each instar, larvae stop feeding and cast off old skin in 18-30 hours. When the worms set for moulting, paraffin paper should be removed and spread on the bed to dry up. If there are more feeding worms, a light and thin feeding may be given. All the worms settle in 6-8 hours. During moulting, worms should not be disturbed and full ventilation should be provided. Feeding is resumed when 90 per cent of worms have moulted. RKO powder is dusted over the worms 30 minutes before feeding. After two consecutive feedings, the larvae with the net are transferred to a new tray. Mature larvae stop feeding and prepare themselves for spinning. Its body becomes translucent, shrinks in length and constrictions appear on fourth and fifth segments. They move towards the periphery of the trays. Such worms are picked and transferred to Chandrika / Netrakae. About 1000 worms (400-450 larvae/m2) can be mounted in a mountage. Mount the entire larvae within a maximum period of 48 hours and provide sufficient ventilation during spinning. Cocoon should be harvested on the fifth and sixth day after mounting. In rainy and cold seasons, it should be delayed for one more day. The cocoons are collected from Chandrika and transported in light gunny bags to cocoon market. The cocoon should be marketed immediately after harvest, so as to avoid adult emergence. Under average conditions, 100 dfls of bivoltine will yield 40-60 kg cocoons and cross breed will yield 30-50 kg cocoons.
Diseases
Pebrine
It is the most destructive disease of silk worms and is caused by protozoa, Nosema bombyscis. The worms become inactive with poor appetite, the skin becomes wrinkled and moulting becomes irregular.
Flacherie
It is caused by bacteria and poor rearing conditions like high temperature, high
humidity, poor ventilation, bad leaf quality, over
feeding etc. aggravates the disease.
Digestive and circulatory systems are damaged and the symptoms are loss of appetite and
diarrhoea.
Grasserie
Mostly seen in ripening larvae. Caused by Borrelina virus. Infection is induced by
extreme low and high temperature.
Swelling of the inter-segmental region,
shining skin, rupture of body wall, oozing
of body fluid and endless crawling are
symptoms. Such worms do not moult and spin.
Muscardine
The fungi Beauveria bassiana, Spicaria prasina and Isaria farinosa are the causal agents. The infected larvae lose appetite. Specks of oozing oily substance without any clear-cut margins appear on the skin. Body generally hardens and becomes stiff.
Prevention and control
1. Disinfect the rearing room and equipment before rearing.
2. Use only disease free layings from authorized agencies.
3. Dip the egg cards in 2 per cent formalin solution for 20 minutes before incubation.
4. Collect undersized larvae and
destroy regularly by burning or burrowing in soil.
5. Feed good quality leaves of correct stages.
6. Avoid over feeding and under feeding
7. Clean the bed every day and burn the infested litter.
8. Use RKO powder at every moulting before resumption of feeding.
9. Maintain humidity only to the desired level.
Pests
Uzi-fly (Trycholyga bombycis)
It is a serious parasite of silkworm larvae and pupae causing heavy loss. Adult is a
large fly with prominent black and grey stripes.
The fly prefers later instars to the earlier ones
for oviposition.
Management
Prevent the entry of fly into the
rearing room by providing wire mesh or nylon net
on doors and ventilators. Burn the parasitized
larvae. Apply chlorpyrifos on the ground and crevices of walls of rearing house.
Other pests include ants, lizards, rats, squirrels,
dermestid beetles and birds.
(Ad hoc
recommendation)
Rats are important non-insect pests
and can be grouped into two different groups as domestic rats and field rats.
Domestic rats
These are found near human dwellings.
1. House rats
(Rattus rattus): There are two subspecies; one with white belly and
the other with grey belly. Tail length is
more than the length of head and body. They are found in houses and eat anything that
man eats. They also cause qualitative damage by
deposition of faecal matter, urine and hairs. It damages gunny bags, plastic
containers, clothes, electric wires etc. House rats 2. House mouse (Mus
musculus): Fur is short without spines. Tail is almost
naked and larger than head and body. The mouse is very active and is found in houses and
gar-dens. It can climb up walls. It damages
clothes, plastic containers and food materials.
3. Large bandicoot rat
(Bandicota Field rats
1. Large bandicoot rat (B.
indica): Large bandicoot rats are also seen in the
field. So this can be considered both as domestic and field rat.
2. Lesser bandicoot rat
(B. bengalensis): It is a short tailed mole rat. Tail length is
only 70 per cent of the body length. Fur is short and coarse. It is seen making burrows in
the paddy field bunds and also in areas where crops like tubers, vegetables, coconut
and young rubber are cultivated.
3. Field mouse (Mus
booduga): Fur is short and coarse and is mostly found in
gardens and fields.Tail is slender and
nearly naked. Tail length is shorter than body and head. The burrows of this species are
found in the paddy fields. They are found feeding on paddy grains in the mature crop as well
as on seeds sown in the nursery.
4. White rat (Tatera
indica): More than one rat per burrow is common in this
species. The eyes are large.The tail is
longer than the body and is provided with a
terminal tuft of long hairs. It is double coloured.
5. Long tailed tree mouse
(Vande-leuria oleracea): The fur is soft and tail
is much longer than the body. They are found in most parts of India inhabiting trees
and shrubs. They damage the inflorescence of arecanut and leafy vegetables by cutting
its leaves. 7. Soft furred field rat
(Millardia meltada): These rats are found in
cultivated field in pairs or small groups of 5 or 6.
They are soft furred without spines. These rats
cut the rice plants in the transplanted crop. The damage starts at the time of planting
and continues up to harvest. The tillers are cut
at the water level. Methods of control In this method of control rats are
rendered to a hostile environment in which they
cannot survive. The mud walls in villages may be replaced by thorny hedges thereby
preventing the rats from making burrows. Good house keeping is regarded as the most
economical and effective way of reducing rat population. Proper sanitation should
be maintained by keeping food material
inaccessible to rats in rat proof
containers. The heap of garbage and sweepings in
streets and towns should not be kept for a long
period. Designing rat proof godowns
and other buildings is another step to ensure environmental control.
Poisoning
Three types of poisoning are usually
employed to control rats.
1. Acute poisons are those that can
kill rats with a single dose; e.g. zinc phosphide. 3. Fumigants are gases and are usually
pumped or released from pellets or
tablets put in through burrow entrances. Pre-baiting for 3-4 days consecutively
is necessary to overcome bait shyness. For
pre-baiting and baiting, the same carrier
material has to be used. Crushed
wheat, maize, bajra, puffed rice, popcorn or rice
mixed with a little jaggery and oil are excellent
carriers. To prepare the carrier, 95 parts by weight of cereal ingredient
is to be mixed with 5 parts of jaggery.
For baiting, zinc phosphide is mixed
with groundnut oil and carrier in the ratio 2:2:96 by weight. At each bait station, 30-40 g
of the bait mixture will have to be exposed. The stations may be selected in areas where
rats are frequent, such as areas around kitchen, store and in homesteads. Expose baits in
the evening and collect them in the following morning. Conduct baiting for three successive days.
Repellents
Chemical repellents include malathion and cyclohexamide which are repellents
to house rats. Both field and domestic rats are
subjected to attack by a range of predators,
parasites and pathogens. The predators include cats,
dogs, snakes, owls, mongooses etc. The
practice of rearing cats in house has
been found to adversely affect rat population. The utilization of microbial pathogens has
not proved successful in any part of the world.
Trapping
Trapping is the oldest method of
controlling rodents. Almost any trap will
catch some rats, but the response varies with different species. The rats are easily caught
in cage or box, but a rat trapped in such trap will be exposed to other rats which
develop trap shyness and they avoid such type of traps. The most effective rat taps
are those, which can completely conceal the rats trapped in it; e.g.
Moncompu trap. The rat traps can be grouped into a few categories. 1. Automatic traps: These have counter
balanced entrances. When an animal
enters this type of traps, its weight
makes it fall into a cage below. The counter
balance on the trap door brings it
back into place, leaving the rodent in the cage. These are intended to catch more
than one rat; e.g. wonder trap.
2. Remote triggered trap: These work by upsetting a delicate balance when the
bait stick is disturbed or when the weight is put on a treadle. Common type of
this is the box or cage trap that captures one rat at a setting. A box trap is a
wooden or metal box open at one or both ends, having one or two doors. Some have
one or both will have overhead trigger on which bait is fastened and the door is
released when the rat works on the bait. Others have a treadle in the floor on
which the rat steps to drop the door
3. Glues: A form of trapping in which a
sticky substance entangles the animal.
4. Pot traps: These traps are extensively
used for catching rice field rats. This trap
consists of a wooden plank, a mud pot
of 10 inch diameter, a metal strip which carry bait and a `Y'shaped wooden
peg to which needle is tied; e.g. Moncompu
trap. 5. Snap traps: Most of the rat traps
fall within this category and are widely used for trapping rats. These kill the rat
instantly by snapping shut when the rat nibbles at the bait placed in the middle of the
open trap. These are variously called as
"break back traps", "guillotine", "spring
traps", "saw toothed traps" and "bamboo traps"
depending upon the materials used in
making them.
6. Kerosene tin trap: It is made by
cutting the top of the tin and filling it with
water up to 15 cm from the top. Chaff is floated on the water surface so that the rat
cannot see water. Attractive and strong
smelling bait like dry fish, fried
coconut etc. is pinned on to a piece of cork or lightwood and floated on the chaff. A
plank is leaned against the side to enable the rat to climb to the top. Seeing no water
and eager to get the bait the rat jumps on to the chaff and gets drowned.
Success or failure of trapping is
dependent up on the following factors.
a. Placement: Traps must be placed
where animals will regularly encounter them.
b. Concealment: It is not advisable to
use new shining traps against rats. To overcome trap shyness it may sometimes
be necessary to cover the trap with a slight coating of
paper or dry leaves that does not interfere with the trigger or action. d. Mechanical conditions: Putting out
traps that are in poor working conditions is a waste of time and effort. f. Bait used: Fresh aromatic bait that is
most attractive to the largest species should be used. Food grains in the houses should
be properly covered so that the rat finds only the food in the trap.
Trapping is the preferred method of
control in the houses and office building,
because animals that get killed can be easily
removed. Traps can be used profitably to
deal with poison-shy and scattered survivors of poison campaign.
Control of important species of rats
BIOCONTROL OF SALVINIA
(Salvinia molesta)
Release of Cyrtobagous
salviniae
weevils is found effective for the control
of salvinia. Even one pair of weevil is sufficient for establishment in a locality. But for practical consideration 50 to 100 weevils
are recommended for release in a
particular area. When collection of weevil is not
possible, about one kg of infested salvinia can be used as the inoculum. Release may
preferably be made whenever tender salvinia growth is available. If the plants are very
old, they may be removed mechanically to
promote re-growth and then weevils are to be released. Almost 100
per cent control of the weed will be obtained in a span of
12-18 months.
The rate of natural dispersal of the
weevil is rather slow and hence it is
desirable that the infested weed mats are
redistributed at periodic intervals. In canals used for navigation, the rate of spread of
the weevil is found to be quite adequate.
BIOCONTROL OF PAPAYA MEALY BUG USING PARASITOID
Papaya mealy bug _ Paracoccus marginatus (Pseudococcidae) : Papaya mealybugs colonise lower side of the
papaya leaves along the veins and later cover the fruits. Due to the mealy bug infestation,
the younger plants are killed outright.
Biological control: Acerophagus
papayae (Encyrtidae) is a parasitoid imported from Puerto Rico. The parasitoid successfully
suppressed the mealybug population in Kerala. The parasitoid is very specific
and did not colonise other species of mealybugs. Release rate is 25 to 50 numbers per
plant. The parasitoid is parasitizing the second instar nymphs of mealybugs. It is very
active and have good host searching ability and suppress the mealybug within 3 to 4
months depending upon the size of the colony.
Mass production of the parasitoid:
The parasitoid can be successfully mass produced in the laboratory on papaya mealybug colonies grown on potato sprouts and
shoots. Two month old potatoes are procured,
washed in water, disinfected using 5% Sodium
hypochlorite solution. Make slight cut on the potato and treat in Giberelic acid 100
ppm solution for half an hour. After air drying
keep the potatoes on sand and cover with black cloth for germination. Good sprouts are
produced within two weeks for mass culturing mealy bugs.The parasitoids are then
released to the colonies of the mealy bug.
A. papyae has very less pre oviposition
period, sex ratio is 1:1, fecundity 50-60, Total life cycle is completed in
16-20 days.
Water hyacinth in water bodies can
be managed by spraying 5 per cent Cashew Nut Shell liquid (CNSL) emulsion followed by spraying 40 per cent Wetable Powder
formulation (WP) of Fusarium pallidoroseum (5
per cent). Spraying may be repeated with WP 5 per
cent alone, after 2 weeks if any new sprouts develop. To prepare 10 litres of 5 per
cent CNSL emulsion, 500 ml of CNSL and 50 g bar soap are required. Slice the bar soap and dissolve
in 500 ml of water. Pour 500ml.of CNSL slowly and stir vigorously to get a good
emulsion. Dilute this one litre solution by adding 9 litres of water to get 10 litres of 5 per cent CNSL emulsion.
* A minimum of 30 minutes may be
given between the application of CSNL and Fusarium
pallidoroseum.
* In moving water bodies fencing with
rope and coconut leaf is recommended.
BIOCONTROL AGENTS AGAINST PLANT PATHOGENS Arbuscular mycorrhizal Fungi (AMF) Trichoderma
Biocontrol of soil borne plant
pathogens involves mass introduction of antagonistic
microorganisms in the soil. Trichoderma
spp. is a group of broad-spectrum antagonists
subjected to detailed studies for their potential as biocontrol agents. They are
effective against the quick wilt of pepper (T.
viride T6, T. longibrachiatum T2), rhizome rot
of cardomom (T. longibrachiatum T2, T.
virens T9) and ginger (T. viride
T10). A non-axenic system, viz. neemcake-cowdung mixture
is used as food base for Trichoderma spp. Trichoderma
harzianum is recommended by fortifying 50kg farmyard manure or
neem cake with 1kg of the mother culture and incubated for 10-15 days before
application in the field (@ 1kg/vine). The mother
culture in liquid formulation can be incorporated
with sterilized coir compost @ 1l/20kg and apply @1kg/vine as above.
(ad hoc recommendation) Two isolates of Pseudomonas
fluor-escens (P1 and P14) have been developed by the Kerala Agricultural University for
the disease management and growth promotion of crop plants. This is found highly
effective for the management of foot rot and
fungal pollu of black pepper, sheath blight and
bacterial leaf blight of paddy, bacterial leaf spot and
Phytophthora infestation in betel vine, bacterial wilt of solanaceous
vegetables, bacterial leaf blight of anthurium and
Colletotrichum and Phytophthora
infestation in vanilla and rhizome rot of
ginger. The organism significantly improves
the growth and biomass production of
crop plants.
Application of Pseudomonas
fluorescens at the rate of 10 g formulation
(1010 cells per gram) mixed with 2 kg of well
decomposed farmyard manure or compost and applied
in the basin of the vine in the field can also
help control foot rot.
Method of application
The time of application and the
frequency of application may vary depending on the crops. The application may be repeated
based on the intensity of the disease incidence.
The talc-based formulation at 1-2
per cent level may be used for soil drenching and
spraying. Seedlings/cuttings are treated with
Pseudomonas culture by dipping the root/tip of cuttings in slurry of
Pseudomonas (250 g
in 750 ml for 20 minutes). For seed
treatment in paddy the talc based culture may be added to the water used for sprouting at
the rate of 10 g per kg of seed.
For transplanted crop, root dip
treatment at the time of transplanting, followed by
a spray 30 days after transplanting. For black pepper, drenching the nursery plants
immediately after planting followed by one or two sprays depending on the extent of
disease. For managing foot rot of pepper in the
main field, drenching the base of the vine and spraying the plant with
Pseudomonas
culture at the rate of 10 g/litre at the
onset of monsoon can be practised. A second spray may be given, if necessary, during the mid-monsoon period.
Chemical fertilizers and plant
protection chemicals should not be used along with biocontrol agents.
Solarization is a method of
hydrothermal disinfection. This is done by covering
moist soil with transparent polythene sheet and exposing it to direct sunlight during the
hottest period of the year.
Methods of solarization
a. Nursery bed
The nursery bed for raising seedlings
is to be levelled and pebbles present on the
surface removed before solarization.
Incorporate the required quantity of organic manure in the soil and irrigate at the rate
of 5 litres per m2. Cover the beds with
100-150 gauge transparent polythene sheets. Seal
the edges of the sheet with soil to keep it in
position in order to maintain the
temperature and moisture inside the polythene mulch.
Adequate care is also to be taken to see that the sheet is in close contact with the
surface of soil to prevent the formation of air
pockets between the soil and polythene sheet. Keep the sheet in this way
for 20-30 days. Protect it from stray animals and birds.
After the period of solarization, remove
the sheet and the bed is ready for sowing and transplanting.
b. Potting mixture
The required type of potting mixture is
to be prepared as per the recommended
practice. Spread this mixture on a levelled ground to a height of 15-20 cm. Moisten
with water using a rose-can and cover the soil
with polythene sheet and solarize for 20-30 days as described above. After solarization, the
soil can be used for sowing/planting. This method is found to be very effective to raise
disease free pepper cuttings. incorporated in soil after removing
the polythene sheet. 1. Solarization is to be done in open
field without any shade.
2. Transparent thin polythene sheet
(100 to 150 guage) is to be used, as it is both cheaper and more effective in heating due to better radiation
transmittance than thicker sheets. 5. Solarization period may be extended to one month or more to ensure pathogen
control at deeper layers. 7. Potting mixture should never be
heaped and solarized, as this will drastically reduce the efficiency of the technique. 2. Control of nematodes: Population
reduction of nematodes like Meloido-gyne, Heterodera,
Xiphinema, etc. can be achieved by solarization. 3. Control of weeds: A number of commonly occurring weeds particularly annuals
can be effectively controlled by solarization. These include, among
monocots, Cynodon dactylon, Cyperus rotundus and Digitaria
ciliaris and among dicots, Crotalaria
muconata, Indigofera hersuita and
Noxia sp. 4. Plant growth response: Increased growth response is observed in plants
cultivated in solarized soil. This is mainly evident
as increase in plant height, number of leaves, better root formation, increased
root nodulation in legumes and yield. MUSHROOM
Cultivation
Species of Pleurotus, commonly
known as oyster mushrooms, grow saprophytically under natural conditions on trees, dead
wood, stumps and branches. Today several species of Pleurotus are commercially grown in many parts of the world. The tropical
climate prevalent in the state is ideal for
mushroom cultivation. Species of Pleurotus
and Volvariella can be successfully cultivated
in the State all round the year on a variety of agro-wastes like saw dust, vegetable
and paper wastes, oil palm pericarp waste and straw. But the most suitable substrate is
found to be paddy straw. Method of cultivation
Polythene bags or tubes of 30 cm x 60
cm size and 150-200 gauge thickness are taken for filling the substrate. If the tubes are
used, the free-end is tied with a string. Seven to eight holes of 0.5-1.0 cm diameter are
made all over the bag for aeration. One kg of well dried, one year old paddy straw is cut
into small bits of 5-8 cm in length and immersed in water for 18 hours. Then the soaked
straw is taken out from water and kept inside the basket for 1-2 hours to drain away
excess water. The soaked straw is kept under
boiling water (100ºC) for 30-40 minutes for
surface sterilization or to achieve
pasteurization and then taken out and kept inside
the basket to drain excess water and is
allowed to cool to room temperature. The
pasteurized straw is ready for filling the
bags. Instead of straw bits, small round straw bundles of 20 cm diameter are also
used for filling the bags. This method is
followed to save time and labour. Now the perforated polythene bag is filled for about 5 cm height with the above processed
straw and pressed with hand for making it even. Care should be taken to fill the bags as
compactly as possible for the proper
growth of mycelium. For getting maximum yield,
2-2.5 per cent (125 g) of spawn is
used. Spawn is taken out from packets and kept inside a clean container or paper. From
this, one tablespoon full of spawn is sprinkled
over the filled straw around the peripheral
region. A second layer of processed straw is
filled and spawned as above. Repeat the process as above until the soaked straw is
finished. Every time before spawning, press the
straw with hand for making it compact. If bundles are used for filling the bags care should
be taken to keep the bundles inside the bag as compact as possible without leaving
any space in between the bundle. Finally the bag is closed tightly with twine and beds are
kept undisturbed for spawn running for about
15-20 days inside the rooms, thatched
rodent-proof sheds or in verandas. The best
temperature and humidity for spawn running ranges from 28-30ºC and 80-85
per cent
respectively. The beds can be arranged over a platform or in shelves. The spawn
running can be judged from the whitish growth
covering the straw completely.
Periodically observe the beds and discard the
contaminated ones.
After 15 days when the
spawn running is complete, remove the polythene bag by cutting it with blade and keep the
bed for sporocarp formation. The opened beds are kept in well-ventilated
rooms. Relative humi-dity of the room should be 80-85
per cent. If temperature inside the room rises above
30ºC, the room should be sprinkled with water
to lower the temperature. Diffused light is essential for normal fruiting. Pinhead
formation starts on 20th day and 2-3 days are
required for the maturation of the fruiting body. Matured and fully opened sporocarps are harvested by placing the thumb and
forefinger near the base of the fruiting body and twisted in clockwise direction to
get it
detached from the mycelium. An average
yield of 500-700 g can be harvested from 1 kg of straw. The spent straw can be used as
enriched cattle feed. • Maintain the
pH of the water used to soak the substrate at 8.0 by adding lime. •Spray 2 per cent
garlic in and around thevicinity of mushroom beds • Spot application
of Carbendazim (at the rate of 50 ppm) in mould affected partsof the bed. • Erect Yellow Light
traps for every 25m 2 at a height of 60 cm from the ground in the mushroom
house. • Hang an yellow
bulb (15W) in between two card board pieces (15 cm x15cm size) coated with
mustard oil. Switch on the bulb from 5 pm to 8 am. Remove insects trapped on
the sticky surface everyday.
Cultivation of paddy-straw mushroom (Volvariella
volvacea) Instead of twists, the beds can be laid out using small bundles of straw each
weighing about one kg. Place four such bundles of straw side by side over the platform with
loose ends towards the same direction. Over this, place another four bundles, the loose
ends towards the opposite direction. These eight bundles form one
layer, which is to be spawned as in the case of twists.
TISSUE CULTURE PROPAGATION OF CROPS Plant tissue culture is the in vitro
culture of plant cells, tissues and organs under
aseptic condition in defined or semi-defined media. Tissue culture techniques are
increasingly being used for the rapid vegetative propagation of plants. It helps in the
mass clonal propagation of crop plants. It is
useful for plants which do not set seeds or where the viability of the seeds is poor.
Even when conventional methods of vegetative propagation are commercially acceptable,
tissue culture propagation can be adopted as it has definite advantages. It offers an
extremely rapid rate of multiplication. The geometric progression of tissue culture
propagation makes it possible to produce millions of plants from an initial explant in a
few months. It can speed up the process of establishing new varieties. Only a limited
quantity of plant tissue is required as the
initial explant. Tissue culture propagation ensures the availability of plants
throughout the year. It helps in the production of
uniform progeny from cross-pollinated
plants. Disease free planting materials can be
made available to the farmers. Special laboratory facilities and technical skill are essential
for adopting this technique for mass multiplication of crop plants. Training in tissue
culture is offered by various research
organizations in Kerala.
Procedure The tools used in the airflow cabinet
may be kept dipped in 70 per cent ethanol in a beaker and periodically flamed over a
spirit lamp. After inoculating the explants in
suitable culture media, the cultures are
incubated in rooms under controlled
conditions of temperature (26 + 2ºC),
light (200 lux, 18 hours) and humidity (60-80 per
cent). Response of an explant largely
depends on the composition of the culture
medium. There are several basal
media, which can be used for various needs with necessary modifications. The basal
medium is selected to suit the plant species and
the method of in vitro culture. In general,
culture medium consists of salts of major and
minor nutrient elements, vitamins, amino acids,
plant growth substances and a source of carbon. The established cultures are sub-cultured
to fresh media at intervals of 3 to 5 weeks. The
media provided at each subculture decide the response of the tissue. Hardening the
plantlets to make them adapt to the outside
environment is a critical process,
essentially due to the anatomical and physiological
peculiarities of the plantlets. A period of
humidity acclimatization is necessary for
the newly transferred plantlets to adapt to the outside environment, during which the
plantlets undergo morphological and
physiological adaptations, enabling them to
develop typical terrestrial plant-water
control mechanism.
Tissue culture techniques for mass
multiplication have been standardized
for crops like banana, pineapple, papaya, black pepper, cardamom, vanilla, orchids,
anthurium, gladiolus and several medicinal plants. The commercial adoption of tissue
culture clonal propagation is feasible only when
the rate of multiplication is satisfactory and the cost of plantlets is acceptable to the
farmers. Protocols for the tissue culture propagation of a number of crops like red
banana, nendran, pineapple, orchid and anthurium, black pepper, vanilla, medicinal plants
etc. have been developed at the Kerala Agricultural University and are available for
commercial adoption.
Improving the KEEPING QUALITY OF (Ad hoc recommendation)
About 30 to 40 per cent of the
harvested fruits and vegetables are estimated to be
lost due to improper harvesting, handling, storage and transportation in India. If proper care
is taken during these operations, the loss can be minimized to some extent. Some of
the techniques, which can be adopted, are as
follows. a) Harvesting must be done at the
appro-priate maturity depending up on the
marketing distances and purpose.
b) Harvesting must be done preferably in
the morning hours or late evening to avoid
exposure of the produce to excessive heat, which will hasten spoilage.
c) Harvesting must be done preferably
with proper harvesting devices suited to the commodity. For example, mango
harvesters with cutting edges and plastic net can prevent the damage during harvest
and collection.
d) Avoid impact shock while harvesting
fruits from tall trees; eg., jackfruit, mango, etc. which will cause bruising, leading to
infection.
e) Avoid too loose or too tight packing
in gunny bags while transporting harvested
produces to minimize bruising. Packing
a) Wash the harvested produce in plain
water or in chlorinated water to clean
it of the adhering mud, dirt and residual
pesticides.
b) Remove the infested, rotten and spoiled
friuts.
c) Grading the produce can improve
market acceptability. This can be done
at farmer's level or at collection points to suit the standards established by
individuals, industry or government. Grading will also increase farmer's bargaining
power, as they are likely to get premium prices for better-grade products. Similarly
buyers can choose the grades they
wish to buy. Possible grading can be based on colour, shape, size, weight etc. of the commodity
a) Pre-cool the commodity immediately
after harvest to reduce the field heat. Some of the packaging techniques are (1) Packing of banana hands at 0.2 to 0.4
per
cent ventilation with polyethylene cover
of 150 gauge can increase the keeping quality upto 10 to 12 days under ambient
conditions. (2) Packing fresh mushroom
(Pleurotus sp.) in 100 gauge polypropylene pouches
without any ventilation can extend the storage life
upto 36 hours at room temperature and up to 7 days under refrigerated conditions. (3)
Fresh tomato can be stored up to 25 days under ambient conditions when packed with 35
to 40 per cent moistened saw dust in the ratio of 1 : 0.5 (tomato : saw dust). (4) Fresh
mature and ripe sapota can be stored up to 6 days under ambient conditions when
individually wrapped with cling film. General storage methods practised to
extend the keeping quality are:
1. By storing the commodity under
optimum/ low temperature and humidity.
2. By skin coating using wax emulsion
containing permitted fungicides at
optimum concentrations. 4. By sub-atmospheric pressure storage.
LOW COST TECHNOLOGY FOR IRRIGATION
(Indigenous auto irrigator for irrigating potted plants)
Indigenous auto irrigator can be
fabricated by fitting certain low cost accessories in
ordinary garden pot. First of all plug the
holes of the garden pot with corks provided with
holes. Insert hospital drips through
these holes. Garden pots designed in this manner can serve as auto irrigator. One auto
irrigator can serve as water source for a
maximum of six pots. Place the irrigator at
a level
above plant height and arrange the potted plant around this auto irrigator. Plants
are irrigated by exploiting gravitational force.
Adjusting the regulator attached to the
hospital drips can regulate the flow of water. Irrigate the pots to bring it to field
capacity. Daily loss of water from the pots can be
computed. The flow rate can be adjusted
according to water requirement of the plant.
KAU Micro sprinkler
ornamental plants etc. have been found
to respond well to this system of irrigation with maximum efficiency.
Low cost greenhouse for protected
cultivation
Naturally ventilated greenhouse made
of bamboo/arecanut/GI pipes and covered with UV stabilized polyethylene sheet are
suitable for growing high value crops like
cabbage, cauliflower, capsicum, tomato and
cucumber round the year. Temperature and humidity build up inside the green house can be
controlled by natural ventilation through
insect proof nets (40-50 mesh) and by
providing the required height to the
structure. The optimum height of a greenhouse
depends on floor area, ambient temperature,
relative humidity, solar radiation and wind velocity
of the locality.
Design of low cost greenhouse
damage tender coconuts and cocoa pods in
the fields. Tey also act as carriers of
several human and animal diseases.
indica): This is the largest domestic rat. Fur is coarse. Tail length is almost equal to
the body length. Body weight ranges from 750 to 1000 g. It damages all tuber crops. It
also damages concrete buildings by making
burrows under the basement.
6. Norway rat (Rattus norvegicus): These rats are found in waterlogged
areas. This is a medium sized rat with tail more
or less equal to the length of the head and body. These rats damage paddy crop. It cuts
the plants at the base and chews the cut portion. Maximum attack is at the booting stage.
The attack ceases after initiation of flowering.
The damage is usually observed in patches away from the field bunds.
8. Bush rat (Golunda elliotti):
These rats are seen in places near forest area.
They live under bushes in nests. These rats are destructive to coffee plants. They feed
on their buds and flowers. They damage paddy by cutting the plants in dryland paddy areas.
Integrated control of field rats
Rats cause considerable damage to
agricultural practices and other human
possessions in addition to acting as carriers of several human and animal diseases.
Diseases like bubonic plague and weils disease (due to contamination of food by
the urine of rats) are caused by rats. It is necessary that the importance of rat control be
understood by all. An integrated approach
to control rats involves the joint utilisation of
all feasible control measures in a complementary manner to maintain the rat population
at a very low level. Integrated control of field rats involves the following: (a) preventing their entry into a region or a building by
putting up mechanical barriers or treating
with repellents; (b) encouraging predators such
as snakes, cats, dogs, mongooses etc.;
(c) causing death by a variety of methods.
Environmental control
2. Multiple dose or chronic poisons
require repeated ingestion over several
succes- sive days; e.g. anticoagulants.
Zinc phosphide
It is a dark grey powder and its toxic action is due to release of phosphine gas.
When it is ingested, phosphine is released causing
injury to the kidneys, liver and lungs
followed by death after a few hours.
Zinc phosphide is used in food baits containing 2 per cent active ingredient.
Biological control
Live traps (cage or box trap)
The trap is to be set up in rice fields,
after placing the base plank above the canopy level on a specially erected platform,
on poles. The rats attracted by the bait climb over to the base plank and try to
snatch off the bait tied on to the metallic strip. Slight disturbance of
the strip dislocates the wooden needle from the strip slot
and causes the pot to fall down abruptly over the rat. The pot and the plank are
tightly held and removed in that position and immersed in water after inversion for
killing the trapped rat. Since the live
rat does not see the captured ones, they do not
develop shyness against this type of mechanical trap.
c. Size and design: Traps should be neither too small nor too large for the
anticipated catch.
e. Number of traps: Large number of traps relative to the expected size of the
rodent population should be used.
Lesser bandicoot rat: These attacking tuber crops can be easily controlled by
poison baiting in rodent burrows. Firstly, locate
the burrows in the field. Open the burrows to a length of 30 to 45 cm. The rats will
come and close the burrows with soil within 30 minutes. Then it can be again opened
and poison bait can be inserted into the burrow.
From bait preference studies indicate that, prawn powder as the most effective bait.
Dry prawn available in the market is heated and powdered. A few drops of vegetable oil
are added and zinc phosphide 1-2 per cent is mixed with the bait. This zinc phosphide
bait can be put inside the burrow preferably on a dry leaf. No pre-baiting is necessary for
these rats in the garden lands since it has no
bait shyness.
Norway rat: The most effective method of control has been found to be the
Moncompu trap. Firstly, fresh rat-damages in the
field have to be located. The rats have a habit of visiting the same area on subsequent
days. Hence the traps should be placed in such
spots.
Preparation of 5 per cent CNSL emulsion
Inoculation with AMF at the time of planting in the nursery or main field improves
the growth and tolerance of crop against root pathogens, particularly
Phytophthora, Pythium, Rhizoctonia and root
nematodes of black pepper, cardamom, ginger,
turmeric, cowpea, rice and transplanted vegetables.
Dry neem cake and cowdung are to be powdered and mixed at 1:1 ratio to get
a coarse texture and then moistened by sprinkling water. Add the
commercial preparation of Trichoderma spp.
(available in polythene packets) @ 1-2 kg per 100
kg of neemcake cowdung mixture. After thoroughly mixing, cover it with a
perforated polythene sheet or ordinary newspaper
and keep it in shade for 4-5 days for multiplication. Again mix well and keep for three
more days for further multiplication. This preparation is ready for incorporation in the
soil. Cowdung alone can also be used as the food base; but, since neem cake is found to be
a better substrate, a mixture of the two is found better than using cowdung alone. If cowdung alone is used, mixing has to be done at 5
days interval and it will be ready for use only on the
15th day. This Trichoderma
incorporated neemcake- cowdung mixture can be used
in the potting mixture in nursery beds and in the
field; i.e. wherever cowdung is used as a manure.
Fluorescent pseudomonas
Fluorescent pseudomonas are a group of bacteria very effective against disease
incited by species of Phytophthora, Pythium,
Rhizoctonia, Fusarium, Colletotrichum,
Ralstonia and Xanthomonas in various
crop plants in the nursery as well as in the main field.
c. Main field
Solarization can also be effectively used for the control of soil borne diseases in
the main field. The land used for planting is initially prepared to a fine tilth and
pebbles removed. Solarization and planting can
then be done as already described. All the other agronomic practices are to be followed
as per the package of practices recommendations. Biopesticides and fertilizers can be
Hints for solarization
3. Summer months are more suitable for
solarization.
4. Soil should be kept moist during
solarization to increase the thermal
sensitivity of resting structures of soil-borne plant pathogens and weeds, and to improve heat conduction.
6. Summer showers will not affect
solarization. However, excessive
seepage of water into the bed during solarization should be avoided.
8. Soil should be in good tilth allowing
close contact between the plastic sheet and the soil to prevent the formation of air pockets, which will reduce heat conduction.
Benefits of solarization
1. Control of fungal pathogens: Several soil borne pathogens can be controlled by
solarization. This includes fungi like Pythium, Phytophthora,
Fusarium, Rhizoctonia etc.
Variety
Ananthan is a short duration variety of oyster mushroom released from KAU. It
is an inter-stock hybrid of Pleurotus
petaloides with firm flesh, pure white colour and is resistant to pest and diseases. It has
good cooking quality as well as consumer acceptability and can be grown on wheat,
paddy and sorghum straw. On an average, it takes eight days from spawning to harvest. Yield
potential is 800 g per kg straw.

Cropping and yield
Management of Pests and contaminants of Oyster mushrooms in Kerala
• Cover the holes with cotton or alternatively put
30 - 40 pin pricks on the polythene cover of the mushroom
bed.
The paddy straw mushroom can be
successfully cultivated in the plains of Kerala throughout the year where the
temperature ranges between 28-32ºC. The straw beds
can be laid out in sheds, veranda of buildings and even under shades of trees during
summer. Beds should not be kept under direct
sunlight. Prepare a raised platform of 1
m long and 0.5 m broad with wooden planks or bricks. Ten to fifteen kg of well-dried
and hand-threshed straw is required to raise a single standard bed. For spawning this
bed, two bottles of spawn and about 100 to 150 g of red gram powder are needed. First
the straw is made into twists of about 5 to 8 m long and 20-25 cm diameter. The twists
are tied into small bundles and are kept immersed in clean water in tanks for about 6 to 12
hours. After this, the bundles are taken out and
kept aside for some time to drain the excess
water. The bundles are untied and the
straightened twists are placed length-wise over the platform in a zigzag fashion.
The twists are placed as close as possible. Keep another layer over the first layer crosswise.
These two layers form the first layer to
be spawned. Break open the spawn bottles and carefully divide the spawn into small bits
of 2-2.5 cm thick. Place these bits of spawn all the rate of along the periphery of the
bed, about 5-8 cm away from the edge and 10 cm apart. Sprinkle a teaspoon full of
coarsely powdered red gram powder before and
after spawning the first layer. Build the next layer with one row of twist as done
before and spawn it. Make successive layers until the straw twists are finished. After
placing the last of twists, press the bed
thoroughly from the top in order to drain excess
water. Make the bed as compact as possible and cover with a transparent polythene sheet
to maintain the temperature and relative humidity within the bed. Place another
wooden plank over the bed and keep 4-5 bricks
above the plank to get more compactness. Keep the bed undisturbed for 6-7 days. Slowly
remove the sheet and observe the moisture level of the straw. If the moisture is
excess remove the sheets for half an hour and then cover it again as before. Small white
round pinheads appear all along the sides of the
bed after 7 days and mature into button and egg stage on 9th day. Harvest the mature
sporocarps in egg stage. About 2-3 kg of mushrooms can be harvested from 10 kg
of straw. Cropping lasts for 2-3 days. After the harvest, the spent straw can be sun-dried
and used as cattle feed.
(Ad hoc
recommendation)
Pipette out the required volume of stock solutions of chemicals into a one litre
glass beaker. Add components like sucrose and myo-inositol as solid and allow them to
dissolve. Make up the volume to approximately 950 ml with distilled water. Adjust the pH
to the required value (5.6 to 5.8 for Murashige and Skoog basal medium) with a few
drops of either alkali or acid, using a pH meter.
Add the required quantity of agar and make up the volume to 1.0 litre. Pour the solution
into a glass beaker and heat, while stirring,
until the agar is dissolved. Dispense the medium (5 to 15 ml) in test tubes or
flasks and plug with cotton. Plastic lids or aluminum foil may also be used for the
purpose. Culture jars may be plugged with plastic
lids. Autoclave the vessels containing culture medium for 15 minutes at 1.06
kg/cm2 pressure (121ºC). While using a
pressure cooker, wait for the continuous flow of
pure steam, put the weight and sterilize for
20 minutes. Explants collected from
field grown plants will have to be disinfected
before inoculating in the culture medium.
The explants are washed in running tap
water first and then in soap solution.
They are then surface sterilized and trimmed
using sterile knives. The commonly used
surface disinfectants are sodium
hypochlorite (0.1 to 2.0 per cent for 15 to 30
minutes) and mercuric chloride (0.05 to 0.1 per
cent for 3 to 20 minutes). The efficiency of the
surface sterilant can be increased, by
adding a few drops of surfactants. After surface sterilization, the explants should be
washed with sterile distilled water four to five
times to remove the residues. The explants are
then transferred to the sterile culture media in vessels. This process is called
inoculation. Surface disinfection and inoculation must
be carried out in a laminar airflow chamber. This equipment can filter the air through a
high efficiency particulate air (HEPA) filter of
very small mesh size. This will remove bacteria and fungal spores. The steady outward
flow of filtered air will ensure a sterile zone in
the equipment, suitable for aseptic manipulations. The needles, forceps, blades and
petri-dishes used for the manipulation of explants
should be pre-sterilized.
FRUITS AND VEGETABLES
Harvesting
During storage
b) Pre-packaging the commodity into unit packs can reduce the handling losses.
3. By adopting controlled/modified atmos-pheric storage modifying the
oxygen/ carbondioxide ratio within the package.
5. By ventilated storage using ventilated films/bags.
6. Using evaporative cool chamber
constructed to store temporarily the
harvested produce at the field before
marketing.
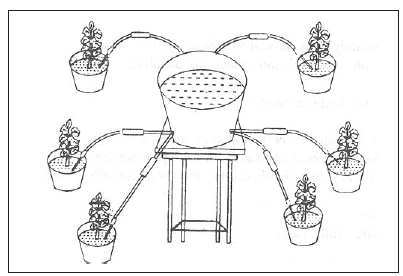
KAU Micro sprinkler is a farmer friendly irrigation system, simple in design, with
less clogging susceptibility, ensuring uniform wetting of the basin of the crops. The
main component of the system is the rotating sprinkler head, made of a small piece of
12mm/8mm dia. LDPE pipe plugged at both ends by end caps. The length of pipe is
6cm for 12mm pipe and 8cm for 8mm
pipe. Nozzles of 1mm diameter are drilled
on opposite sides of the pipe, 5mm away
from both ends, at 900 from bottom. It
is centrally attached to a 6mm micro tube and then to the lateral of the pipe network
through pin connectors. The micro tube
with sprinkler head unit is held erect by
tying to a riser pipe, fixed near the plant to
be irrigated.
The maximum allowable length of
laterals in this system is 50m with about
20 sprinkler heads. An area of 1.0 ha can be irrigated in two splits by a 0.5 to 1.0
hp pumping unit with a pressure of 1.0 to
2.0kg/cm2. The units are capable of discharging 35 to
45 lph with an area of coverage upto 2.5m diameter. Coconut, Arecanut, banana,
vegetables, vanilla, medicinal plants, lawns,


An optimal design of a low cost greenhouse suitable for homesteads of Kerala
is a gable shaped structure with a floor area of
75m2 provided with roof and side
ventilation. The structure should have a ridge height
of 4.35 m and gutter height of 2.5m. The roof slope should be around
300, effective side ventilation not less than 30
per cent and
effective roof ventilation not less than
9 per cent the floor area of the greenhouse. The temperature inside the greenhouse
increase with increase in floor area and
decreases with increase in height of the
greenhouse. Hence height of greenhouse
has to be increased with increase in floor area.
The structure can be made of arecanut/GI pipes/bamboo poles. The
bamboo/arecanut poles should be treated with chlorpyriphos (0. 2
per cent) to prevent termite attack. The structure should be
covered with UV stabilized
polyethylene sheet (200 micron) with at least 85 per
cent light transmissibility. Side ventilators
should be provided on either side of greenhouse at the floor level and roof ventilators should
be provided at the top level throughout the length of the greenhouse as shown in figure.
Ventilators should be provided with insect proof net. Crop yield under the naturally
ventilated greenhouse is generally 3.5 times more
than that of open field. Insect and other pest
attack is limited to thrips and mites for which suitable control measures should be
adopted. Off season production of vegetables is
also possible in greenhouse which fetches a high market price to the farmer.
Citation:
Kerala Agricultural University. 2011.
Package of Practices Recommendations: Crops.
14th Edition. Kerala Agricultural University, Thrissur. 360p.